Research Topics
Holzgrabe group: research topics
Medicinal Chemistry
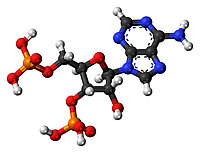
"Medicinal chemistry is a chemistry-based discipline, also involving aspects of biological, medical and pharmaceutical sciences. It is concerned with the invention, discovery, design, identification and preparation of biologically active compounds, the study of their metabolism, the interpretation of their mode of action at the molecular level and the construction of structure-activity relationships." (http://www.chem.qmul.ac.uk/iupac/medchem/ix.html#m1)
We focus on a variety of topics in the field of medicinal chemistry and pharmaceutical analysis, including:
These projects are linked to the Collaborative research center (SFB 630): Recognition, Preparation and Functional Analysis of Agents against Infectious Diseases (see: http://www.sfb630.de/).
Smart and small molecules are aimed to be developed for treatment of tuberculosis, legionellosis, African sleeping, sickness and leishmaniosis. Both ligand- and structure-based drug design will guide the way to innovative active substances.
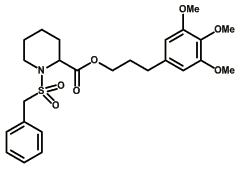
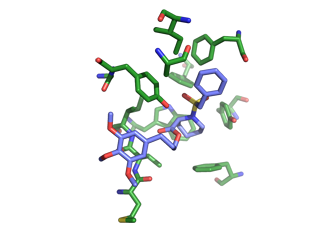
This is most active compound inhibiting the so-called PPIase activity, but does not inhibit the dessimination of Legionella.
- Macrophage infectivity potentiator protein being an extracellullar major virulence factor produced e. g. by Legionella pneumophila.
- Mip contributes to the bacterial dissemination within the lung tissue and the spread of Legionella to the spleen.
- Mip exhibits a peptidyl prolyl cis-trans isomerase activity (PPIase).
- Burkholderia Mip is required for efficient invasion of the host cells and a virulence factor in mouse models.
This is most active compound inhibiting the so-called PPIase activity, but does not inhibit the dessimination of Legionella.
For details see:
Pipecolic acid derivatives as small-molecule inhibitors of the Legionella Mip Protein
C. Juli, M. Sippel, J. Jäger, A. Thiele, M. Weiwad, K. Schweimer, B. Wöhrl, P. Rösch, M. Steinert, C. A. Sotriffer, U. Holzgrabe, J. Med. Chem. 54, 277-283 (2011)
The aforementioned inhibitor as well as a couple of smaller molecules could be crystallized with Burkholderia pseudomallei (Bp) Mip and show antiinfective activity against the Bp.
For details see: A structural biology approach enables the development of antimicrobials targeting bacterial immunophilins. D.W. Begley, D. Fox, D. Jenner, C. Juli, P.G. Pierce, J. Abendroth, M. Muruthi, K. Safford, V. Anderson, K. Atkins, S.R. Barnes, S.O. Moen, A.C. Raymond, R. Stacy, P.J. Myler, B.L. Staker, N.J. Harmer, I.H. Norville, U. Holzgrabe, M. Sarkar-Tyson, T.E. Edwards, D.D. Lorimer, Antimicrob. Agents Chemother. 58(3), 1458-1467 (2014)
Aims: Optimization of the anti-PPIase-activity and antiinfective activity against Burkholderia pseudomallei; investigation of the mode of action of the PPIase-Mip-inhibition against Legionella
Collaboration partners: Mikrobiology Lg MIP: PPIase-Assay: M. Weiwad, A. Thiele, MPI Proteinfaltung, Halle; Infection assay: M. Steinert, Mikrobiologie, Braunschweig; B. pseudomallei Mip: I. H. Norville; (Exeter, Salisbury, UK); NMR-Spectroscopy Lg Mip: P. Rösch, K. Schweimer, Department Biopolymers, Bayreuth; Molecular Modelling Lg Mip: C. Sotriffer; Protein crystallography and NMR spectroscopy of BpMip: R. Stacy, M. Sarkar-Tyson, D. Lorimer (EmeraldBio, Seattly, USA and University of Western Australia, Perth, Australia)
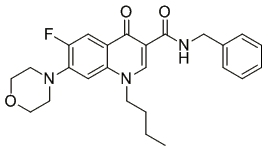
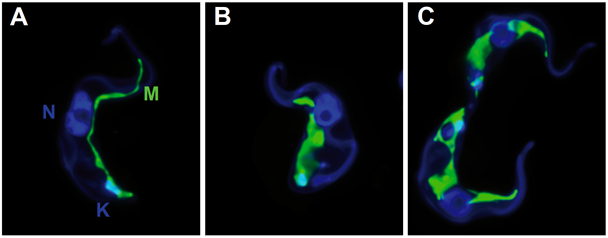
By serendipity, the high activity of the quinolone-carboxamides against Trypanosoma brucei was detected and optimized. The best compound is shown here. Mode of action: change in the morphology of the mitochondrium; most likely the defect in kinetoplast segregation. This mechanism is not identical with ciprofloxacin and thus, the target is not necessarily the topoisomerase.
- Lipophilicity logP 2.4 to 4.0
- Mol. Weight 400 to 550
- Less than 10 H-bond acceptors
- Less than 5 H-bond donors
- Low water solubility
- IC50 (T.b.brucei, 72h) = 47 nM
- IC50 (BSF T.b.b., 48h) = 23 nM
- IC50 (T.b. rhodesiense, 72h) = 9 nM
- IC50 (J774.1 macrophages) = 57 µM
- Cytotoxicity:
The higher trypanocidal activity, the more pronounced the gap to cytotoxicity:SI > 1000 can be easily achieved - Metabolic stability
For details see:
Synthesis and Structure-Activity Relationships of New Quinolone-type Molecules against Trypanosoma brucei., G. Hiltensperger, N. Jones, S. Niedermeier, A. Stich, M. Kaiser, J. Jung, S. Puhl, A. Damme, H. Braunschweig, L. Meinel, M. Engstler, U. Holzgrabe, J. Med. Chem. 55, 2538-2548 (2012)
Aims: Development of compounds with high in vivo activity; improvement of water solubility; complete elucidation of the mode of action.
Collaboration partners: Mikrobiology: M. Engstler, & N. Jones (Würzburg); Pharmaceutics: L. Meinel, A. Sakalis (Würzburg)
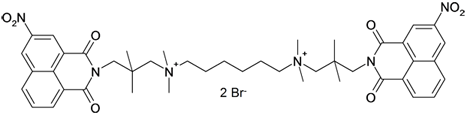
Tertiary bisammonium bisnaphthalimides are known to be cancerostatic. However, the quaternary analogues do not exhibit this cytotoxicity, but are active against tropical protozoa and staphylococci. Of note, the structure-activity relationships of the naphthalamides are different in the different protozoa and Staphylococcus aureus.
For details see e.g.:
Mode of action studies of the novel bisquaternary bisnaphthalimide MT02 against Staphylococcus aureus, T. M. Menzel, M. Tischer, P. François, J. Nickel, J. Schrenzel, H. Bruhn, A. Albrecht, L. Lehmann, U. Holzgrabe, K. Ohlsen, Antimicrob. Agents Chemother. 55, 311-320 (2011)
The Bisnaphthalimides as New Active Lead Compounds against Plasmodium falciparum, M. Tischer, L. Sologub, G. Pradel, U. Holzgrabe, Bioorg. Med. Chem. 18, 2998-3003 (2010)
Antitrypanosomal activity of quaternary naphthalimide derivatives, M. Muth, V. Hoerr, A. Stich, U. Holzgrabe, Bioorg. Med. Chem. Lett. 17, 1590-1593 (2007)
Aims: Optimization of the compounds against the various microorganisms. Bisquaternary bistacrine derivatives show a similar activity. Optimization of this series with regard to antiinfective activity and toxicity.
Collaboration partners: Microbiology: K. Ohlsen (Würzburg), A. Stich (Würzburg), G. Pradel (Aachen); H. Moll & U. Schurigt (Würzburg)
The structures of the human and bacterial enzymes building fatty acids are already elucidated. This holds true inter alia for the elongation-condensation enzyme FabB of E. coli. The corresponding enzyme KasA was isolated from mycobacteria and structurally characterized in presence of thiolactomycin inhibitor. Virtual screening gave suggestions for inhibitors which are synthesized and biologically evaluated by means of a fluorescence assay. Some compounds exhibiting good KasA inhibitory activity were identified which are in part active against mycobacterium tuberculosis.
Aims: Development of an enzyme assay which specifically measures the inhibition of KasA; crystal structure of KasA complexed with a newly synthesized inhibitor.
Collaboration partners: C. Kisker and C. Sotriffer (Würzburg), P. Tonge (Stony Brook, NY, USA)
The extract from the plant collected in India was found to have substantial antileishmanial activity which might be due to an antiprotease activity.
For first results see:
Valeriana wallichii root extracts and fractions, with activity against Leishmania spp, S. Ghosh, S. Debnath, S. Hazra, A. Hartung, K. Thomale, M. Schultheis, P. Kapkova, U. Schurigt, H. Moll, U. Holzgrabe, B. Hazra, Parasitology Res., 108, 861-871 (2011)
Aims: Isolation of the most active compound in the extract and elucidation of the mode of action.
Collaboration partners: Medicinal Chemistry: B. Hazra (KolKatta, India); Microbiology: H. Moll & U. Schurigt (Würzburg); Enzymology: T. Schirmeister (Mainz)

This project is linked to the clinical research unit (CRU 216): "Characterization of the Oncogenic Signaling Network in Multiple Myeloma: Development of Targeted Therapies" (for more information click here ).
Heat shock proteins 70 (Hsp70) are highly conserved molecular chaperones, ubiquitously present in eukaryotic cells. Due to their numerous cellular functions, including correct folding and refolding of proteins, prevention and dissolution of protein complexes, degradation of unstable and misfolded proteins, and the control of regulatory proteins, these enzymes play an important role in protein homeostasis. The family of 70kD chaperones comprises two kinds of proteins, stress-inducible and constitutively expressed isoforms, also known as heat shock cognates (Hsc70). In recent years, increasing evidence has suggested Hsp70 as a potential anti-cancer target. In this project we target the interface between the substrate-binding protein and the nucleotide binding site. By means of virtual screening a hit was found and the correspondingly developed compound library led to a highly active inhibitors.
Aims: Further optimization of the inhibitors, elucidation of the molecular mode of action; development of inhibitors of the HSF-1 protein.
Collaboration partners: C. Sotriffer (Pharmazie), C. Grimm (structure biology), M. Chatterjee & R. Bargou (Medizinische Klinik)
Since a long time we are interested in allosteric modulators of muscarinic receptor. In collaboration with K. Mohr (Bonn) we have developed positive allosteric modulators and have taken the concept to bitopic dualsteric compounds which simultaneously bind to the orthosteric and allosteric site of the muscarinic receptor. Beside receptor subtype selectivity a signalling selectivity was observed.
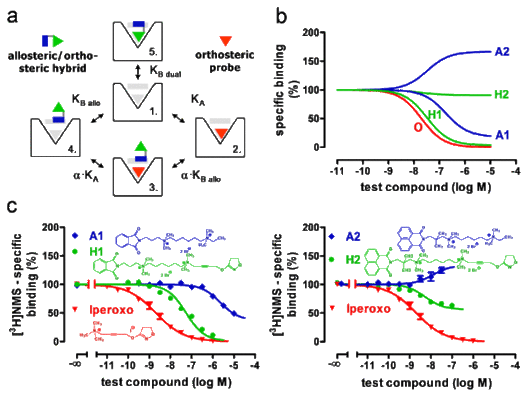
(a) Extended allosteric model including the two possible modes of hybrid binding "4" and "5". K-values: equilibrium dissociation constants of ligand binding; α: allosteric/orthosteric cooperativity factor.
(b) Predicted test compound effects on orthosteric radioligand binding. O: orthosteric building block; A1, A2: allosteric building blocks; H1, H2: hybrid compounds.
(c) Effects of the indicated test compounds on orthosteric [3H]N-methylscopolamine ([3H]NMS) binding to human muscarinic M2 receptors contained in COS7-hM2 cell membranes. [3H]NMS specific binding is indicated in percent of the value without test compound. Curve fitting was based on a four parameter logistic function for iperoxo, on the ternary complex model of allosteric interactions for A1 and A2, on the extended allosteric model for H1 and H2. (Taken from FASEB Journal 23, 442-450 (2009)
For details see e.g.:
Design, Synthesis and Action of Oxotremorine-related Hybrid-type Allosteric Modulators of Muscarinic Acetylcholine Receptors, T. Disingrini, M. Muth, C. Dallanoce, E. Barocelli, S. Bertoni, K. Kellershohn, K. Mohr, M. De Amici, U. Holzgrabe, J. Med. Chem. , 366-372 (2006)Dualsteric GPCR targeting: a novel route to binding and signalling pathway selectivity, J. Antony, K. Kellershohn, M. Mohr-Andrä, A. Kebig, S. Prilla, M. Muth, E. Heller, T. Disingrini, C. Dallanoce, S. Bertoni, J. Schrobang, C. Tränkle, E. Kostenis, A. Christopoulos, H.-D. Höltje, E. Barocelli, M. De Amici, U. Holzgrabe, K. Mohr, FASEB Journal 23, 442-450 (2009)
Extracellular allosteric domain rearrangement pilots orthosterically-induced signaling of a seven helical transmembrane receptor through sequential conformational states, A. Bock, N. Merten, R. Schrage, C. Dallanoce, J. Bätz, J. Klöckner, J. Schmitz, C. Matera, K. Simon, A. Kebig, L. Peters, A. Müller, J. Schrobang-Ley, C. Tränkle, C. Hoffmann, M. De Amici, U. Holzgrabe, E. Kostenis, K. Mohr, Nature Commun, In press
Aims: Elucidation of the mechanism of the signaling selectivity by using the dualsteric ligands as a pharmacological tool.
Collaboration partners: K. Mohr (Pharmakologie Bonn), E. Kostenis (Pharmakologie, Bonn), C. Hoffmann (Pharmakologie, Würzburg)
(a) Extended allosteric model including the two possible modes of hybrid binding "4" and "5". K-values: equilibrium dissociation constants of ligand binding; α: allosteric/orthosteric cooperativity factor.
(b) Predicted test compound effects on orthosteric radioligand binding. O: orthosteric building block; A1, A2: allosteric building blocks; H1, H2: hybrid compounds.
(c) Effects of the indicated test compounds on orthosteric [3H]N-methylscopolamine ([3H]NMS) binding to human muscarinic M2 receptors contained in COS7-hM2 cell membranes. [3H]NMS specific binding is indicated in percent of the value without test compound. Curve fitting was based on a four parameter logistic function for iperoxo, on the ternary complex model of allosteric interactions for A1 and A2, on the extended allosteric model for H1 and H2. (Taken from FASEB Journal 23, 442-450 (2009)
For details see e.g.:
Design, Synthesis and Action of Oxotremorine-related Hybrid-type Allosteric Modulators of Muscarinic Acetylcholine Receptors, T. Disingrini, M. Muth, C. Dallanoce, E. Barocelli, S. Bertoni, K. Kellershohn, K. Mohr, M. De Amici, U. Holzgrabe, J. Med. Chem. , 366-372 (2006)Dualsteric GPCR targeting: a novel route to binding and signalling pathway selectivity, J. Antony, K. Kellershohn, M. Mohr-Andrä, A. Kebig, S. Prilla, M. Muth, E. Heller, T. Disingrini, C. Dallanoce, S. Bertoni, J. Schrobang, C. Tränkle, E. Kostenis, A. Christopoulos, H.-D. Höltje, E. Barocelli, M. De Amici, U. Holzgrabe, K. Mohr, FASEB Journal 23, 442-450 (2009)
Extracellular allosteric domain rearrangement pilots orthosterically-induced signaling of a seven helical transmembrane receptor through sequential conformational states, A. Bock, N. Merten, R. Schrage, C. Dallanoce, J. Bätz, J. Klöckner, J. Schmitz, C. Matera, K. Simon, A. Kebig, L. Peters, A. Müller, J. Schrobang-Ley, C. Tränkle, C. Hoffmann, M. De Amici, U. Holzgrabe, E. Kostenis, K. Mohr, Nature Commun, In press
Aims: Elucidation of the mechanism of the signaling selectivity by using the dualsteric ligands as a pharmacological tool.
Collaboration partners: K. Mohr (Pharmakologie Bonn), E. Kostenis (Pharmakologie, Bonn), C. Hoffmann (Pharmakologie, Würzburg)
The department has a long standing tradition in the development of monographs for the German Pharmacopoeia (Deutsches Arzneibuch) and the European Pharmacopoeia. This is especially true when it comes to the identification, separation, and quantification of the impurity profile of drugs. Employing HPLC-UV; HPLC-CAD, HPLC-ELSD, as well as capillary electrophoresis and sometimes NMR spectroscopy the quality of the drugs can be ensured.
Capillary electrophoresis techniques (CZE, MEKC, MEEKC) modified with various cyclodextrin derivatives are often used for chiral analysis. NMR spectroscopy and molecular simulations were employed for the elucidation of the mode of chiral recognition.
For details see e.g.:
- Comparison of cyclodextrin-dipeptide inclusion complexes in absence and presence of urea by means of capillary electrophoresis, nuclear magnetic resonance and molecular modelling, B. Waibel, J. Scheiber, C. Meier, M. Hammitzsch, K. Baumann, G. K. E. Scriba, U. Holzgrabe, Eur. J. Org. Chem. 2007, 2921-2930
- Enantioseparation of DOPA and related compounds by cyclodextrin-modified microemulsion electrokinetic chromatography, C. Borst, U. Holzgrabe, J. Chromatogr. A 1024, 191-196 (2008)
Control of impurities in L-aspartic acid and L-alanine by high-performance liquid chromatography coupled with a corona charged-aerosol detector, U. Holzgrabe, C.-J. Nap, S. Almeling, J. Chromatogr. A 1217, 294-301 (2010)
Collaboration partners: S. Almeling (EDQM, Straßburg), J. Norwig (BfArM)
qNMR spectroscopy can be used as an orthogonal method for the quantification of impurity in a drug. Parameter setting for measurement and processing is critical for sensitivity and precision of the method. Beside quality assurance of drugs, qNMR is utilized in unraveling counterfeits.
Different NMR techniques, such as DOSY, ROESY, etc., are used for elucidation of counterfeits from drug formulations, for characterization of e.g. ionic liquids and others.
For details see e.g.:
- Quantitative NMR Spectroscopy in Pharmaceutical Applications U. Holzgrabe, Progr. NMR spectroscopy , 229-240 (2010)
Books:
- NMR Spectroscopy in Drug Development and Drug Analysis, U. Holzgrabe, B. Diehl, I. Wawer, VCH-Wiley, Weinheim, 1999
- NMR Spectroscopy in Pharmaceutical Analysis, U. Holzgrabe, B. Diehl, I. Wawer, Elsevier, Amsterdam, 2008
Collaboration partners: B. Diehl & T. Beyer (Spectralservice Cologne), I. Wawer (Warsaw, Poland)
In the beginning of the century we were involved in the gentamicin case. Some 80 people died in the US according to impure gentamicin. Using MEKC and qNMR spectroscopy we could characterize the impurity profile of the dangerous batches. More recently we were involved in the heparin case where heparin was poisoned with oversulfated chondroitin sulfate. We have observed the German market by using qNMR and CZE. In addition biological assays were employed in collaboration with S. Alban (Kiel) and B. Wolff (Ratiopharm, Ulm).
For details see e.g.:
Quality assessment of unfractionated heparin using 1H nuclear magnetic resonance spectroscopy, T. Beyer, B. Diehl, G. Randel, E. Humpfer, H. Schäfer, M. Spraul, C. Schollmayer, U. Holzgrabe, J. Pharm. Biomed. Anal. 48, 13-19 (2008)
Composition of OSCS-contaminated Heparin Occurring 2008 in Batches on the German Market, U. Holzgrabe, S. Alban, K. Baumann, J. Norwig, B. Wolf, D. Brinz, T. Beyer, M. Matz, Eur. J. Pharm. Sci. 40, 297-304 ( )
Collaboration partners: S. Alban (Kiel), B. Wolff (Teva, Ulm), J. Norwig (BfArM), K. Baumann (Braunschweig)